ARTICLE: Zinc Aqueous Battery – Future of Energy Storage
We contributed the computational work to this paper, which was led by our collaborators at RPI.
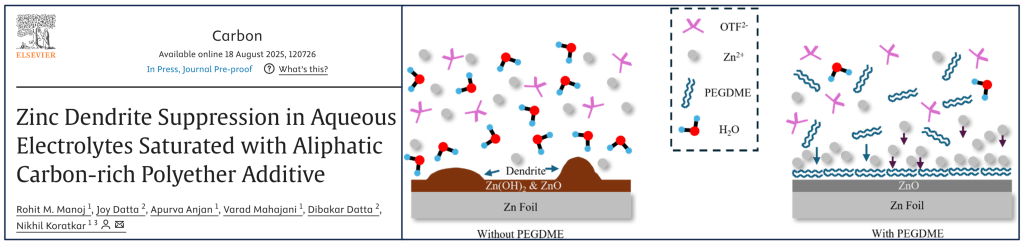
We contributed the computational work to this paper, which was led by our collaborators at RPI.

New Paper in Royal Society of Chemistry (RSC) Energy Advances
CLICK HERE TO ACCESS THE PAPER ; PDF Link
New Preprint : CLICK HERE
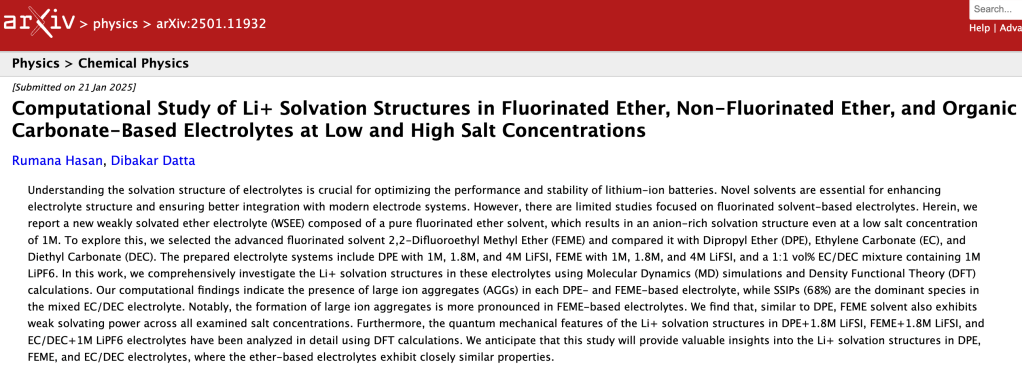
New Preprint : CLICK HERE

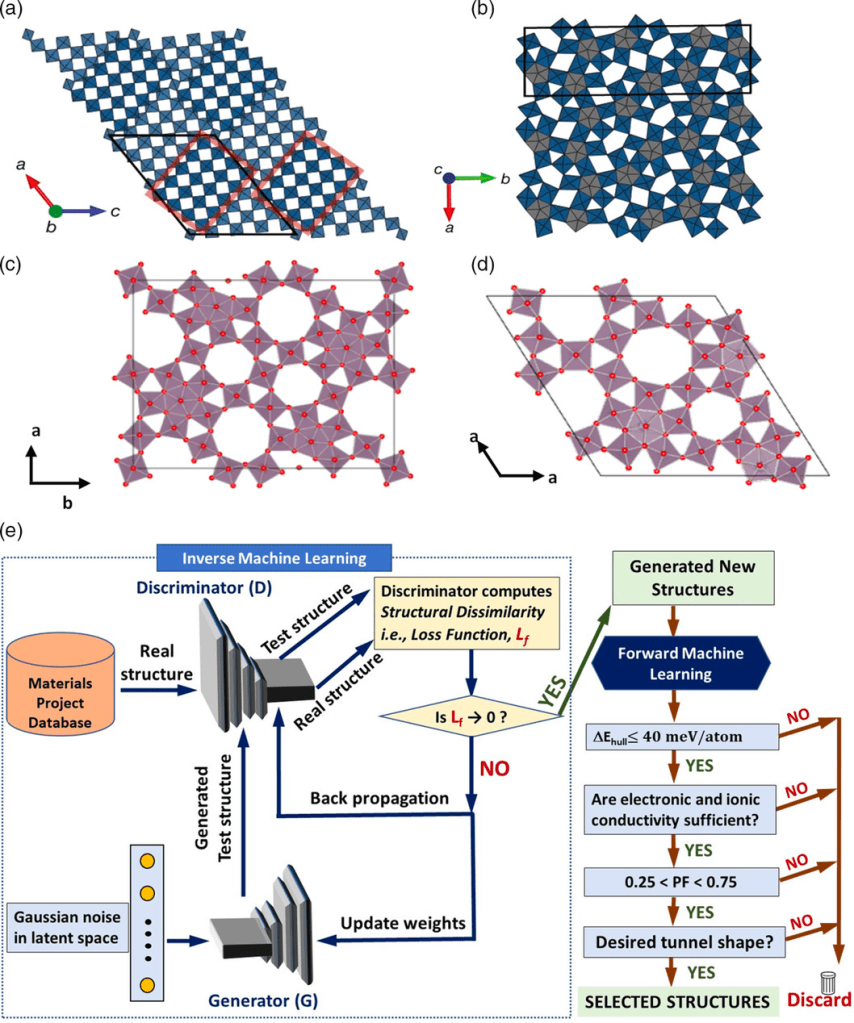
** J. Datta, N. Koratkar, D. Datta, Unlocking the Potential of Open-Tunnel Oxides: DFT-Guided Design and Machine Learning-Enhanced Discovery for Next- Generation Industry-Scale Battery Technologies, RSC Energy Advances
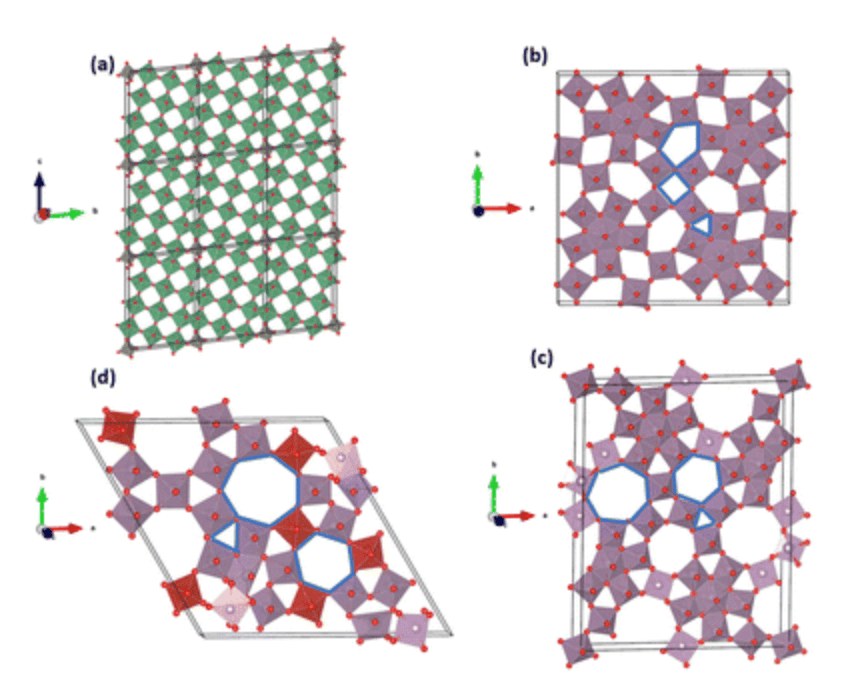
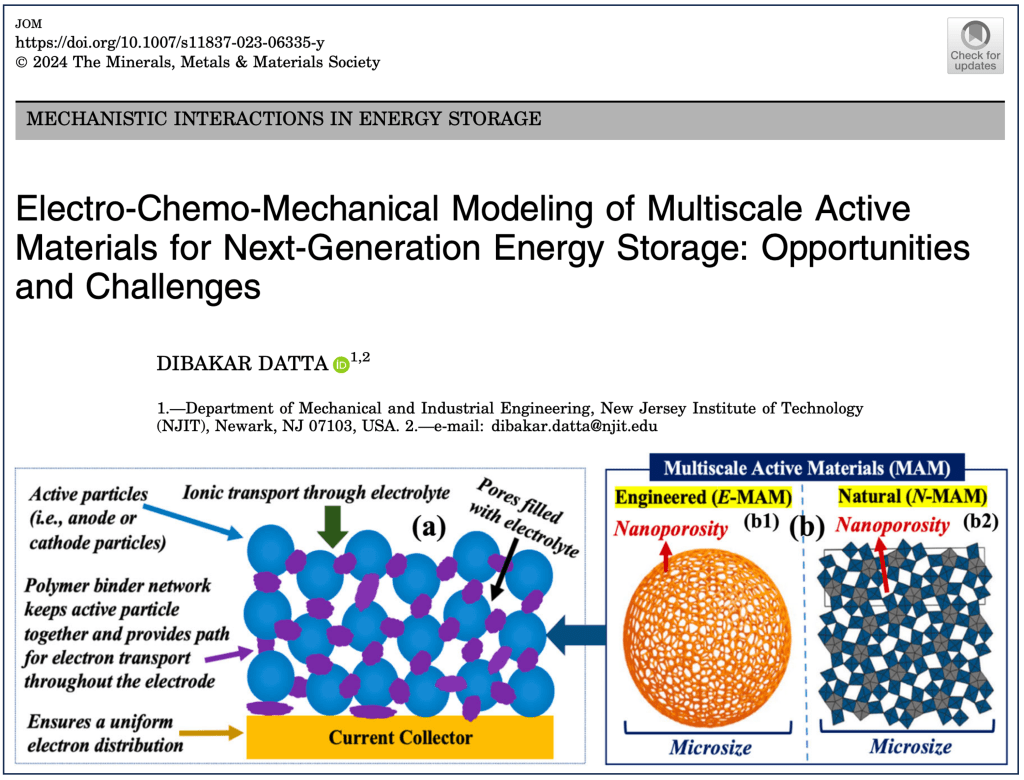
Datta, D. Electro-Chemo-Mechanical Modeling of Multiscale Active Materials for Next-Generation Energy Storage: Opportunities and Challenges. JOM (2024).
LINK : https://doi.org/10.1007/s11837-023-06335-y
Although lithium-ion batteries represent the best available rechargeable battery technology, a significant energy and power density gap exists between LIBs and petrol/gasoline. The battery electrodes comprise a mixture of active materials particles, conductive carbon, and binder additives deposited onto a current collector. Although this basic design has persisted for decades, the active material particle’s desired size scale is debated. Traditionally, microparticles have been used in batteries. Advances in nanotechnology have spurred interest in deploying nanoparticles as active materials. However, despite many efforts in nano, industries still primarily use ‘old’ microparticles. Most importantly, the battery industry is unlikely to replace microstructures with nanometer-sized analogs. This poses an important question: Is there a place for nanostructure in battery design due to irreplaceable microstructure? The way forward lies in multiscale active materials, microscale structures with built-in nanoscale features, such as microparticles assembled from nanoscale building blocks or patterned with engineered or natural nanopores. Although experimental strides have been made in developing such materials, computational progress in this domain remains limited and, in some cases, negligible. However, the fields hold immense computational potential, presenting a multitude of opportunities. This perspective highlights the existing gaps in modeling multiscale active materials and delineates various open challenges in the realm of electro-chemo-mechanical modeling. By doing so, it aims to inspire computational research within this field and promote synergistic collaborative efforts between computational and experimental researchers.
New Paper by Dr. Vidushi Sharma :
Effects of Graphene Interface on Potassiation in a Graphene-Selenium Heterostructure Cathode for Potassium-Ion Batteries, ACS Applied Energy Materials, 2023

Selenium (Se) cathodes are an exciting emerging high energy density storage system for potassium-ion batteries (KIB), where potassiation reactions are less understood. Here, we present an atomic-level investigation of a KxSe cathode enclosed in hexagonal lattices of carbon (C) characteristic of a layered graphene matrix and multiwalled carbon nanotubes (MW-CNTs). Microstructural changes directed by the graphene−substrate in the KxSe cathode are contrasted with those in the graphene-free cathode. Graphene’s binding affinity for long-chain polyselenides (Se3 = −2.82 eV and Se2 = −2.646 eV) at low K concentrations and ability to induce enhanced reactivity between Se and K at high K concentrations are investigated. Furthermore, intercalation voltage for graphene-enclosed KxSe cathode reaction intermediates is calculated with K2Se as the final discharged product. Our results indicate a single-step reaction near a voltage of 1.55 V between K and Se cathode. Findings in the paper suggest that operating at higher voltages (∼2 V) could result in the formation of reaction intermediates where intercalation/deintercalation of K could be a challenge, and therefore cause irreversible capacity losses in the battery. The primary issue here is the modulating favorability of graphene surface toward discharging of Se cathode due to its differential preferences for K−Se reaction intermediates. A comparison with a graphene-free cathode highlights the substantial changes a van der Waals (vdW) graphene interface can bring in the atomic structure and electrochemistry of the KxSe cathode.

Exciting News !! Dibakar won the highly prestigious US National Science Foundation CAREER Award.
Program : Mechanics of Materials and Structures (MOMS)
Project : CAREER: Electro-Chemo-Mechanics of Multiscale Active Materials for Next-Generation Energy Storage
Program Manager : Dr. Wendy Crone
San Diego Supercomputer Center (SDSC) highlighted our work: Graphene, Tin Combo Shows Promise for Solar Panels, Artificial Muscles and More
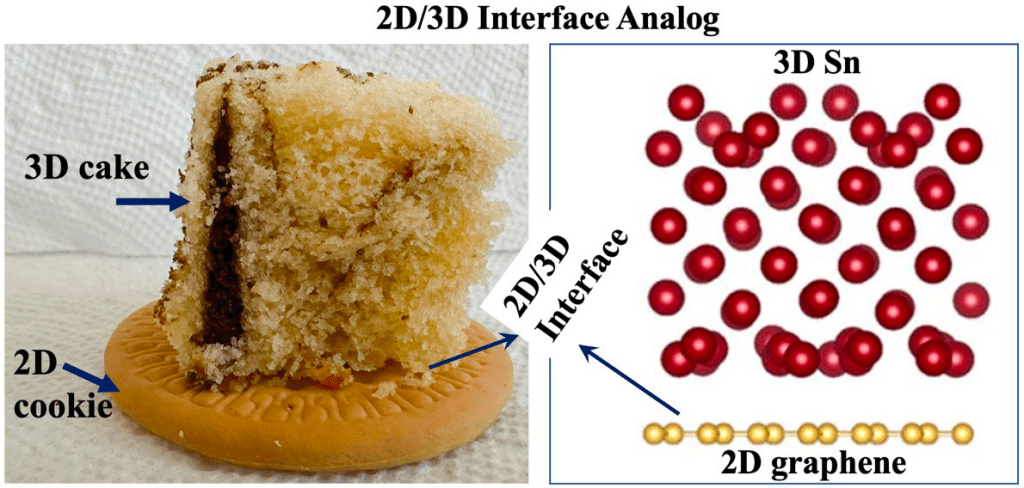
Check the related paper : Developing Potential Energy Surfaces for Graphene-Based 2D–3D Interfaces From Modified High-Dimensional Neural Networks for Applications in Energy Storage, ASME Journal of Electrochemical Energy Conversion and Storage, 19(4):041006, 2022
Among intercalation, alloying, and conversion battery chemistries, the intercalation chemistry is most widely used in commercial applications due to its superior reversibility, round trip efficiency, and stability, albeit at the expense of reduced specific capacity. While intercalation hosts for monovalent ions (e.g., lithium and sodium) are well developed, the jury is still out on the best available intercalation host materials for multivalent ions such as magnesium, zinc, calcium, and aluminum. In multivalent systems, it is challenging to find electrode materials that can act as a durable host, and accommodate large number of ions, while also permitting fast diffusion kinetics. In this perspective, the electrochemical performance of five distinct class of materials (prussian blue analogues, sodium super ionic conductors organic, layered, and open-tunnel oxides) for multivalent ion storage is evaluated. The analysis reveals that open-tunnel oxides show noticeably superior performance in multivalent ion batteries. Herein, the underlying reasons for this are discussed and the case is made for an in-depth machine-learning-driven “materials exploration effort” directed toward discovery of new open-tunneled oxides that could lead to vastly superior multivalent ion batteries.